Carbon has six electrons. It is a fundamental building block of life and found in all organic compounds.
Understanding the electron configuration of carbon is essential in chemistry and biology. With its unique ability to form covalent bonds, carbon plays a crucial role in the structure and function of molecules. From DNA and proteins to carbohydrates and fats, carbon’s versatility is unparalleled.
By knowing the number of electrons in a carbon atom, scientists can predict its behavior and interactions with other elements. This knowledge is vital for various fields, including medicine, environmental science, and materials engineering. By delving into the electron count of carbon, we gain insights into the complexities of the natural world.
The Essence Of Carbon In The Periodic Table
Carbon, a vital element in the periodic table, possesses six electrons. Its atomic number is 6, indicating the number of electrons in its neutral state. Carbon’s unique electron configuration influences its chemical properties and bonding abilities.
Position And Significance
The element carbon is a vital component in the periodic table, holding the sixth position. Its significance lies in the fact that carbon is the foundation of all organic compounds, making it essential for life on Earth. Carbon has the ability to form bonds with other elements, giving rise to an incredible diversity of compounds.
Atomic Structure Insights
The atomic structure of carbon provides fascinating insights into its properties. Carbon has an atomic number of 6, indicating that it possesses six protons in its nucleus. This also means that carbon typically has six electrons surrounding the nucleus.
Carbon’s electron configuration is 1s2 2s2 2p2, which means it has two electrons in its 1s orbital, two in its 2s orbital, and two in its 2p orbital. The 2s and 2p orbitals are involved in forming chemical bonds.
Furthermore, carbon is known for its ability to form four covalent bonds. This characteristic allows carbon to create stable compounds with a wide variety of elements, such as hydrogen, oxygen, nitrogen, and many others. These covalent bonds play a crucial role in the formation of complex organic molecules, including carbohydrates, lipids, proteins, and nucleic acids.
Carbon’s ability to form long chains and complex three-dimensional structures contributes to the immense diversity of organic compounds found in nature.
Overall, the essence of carbon in the periodic table lies in its unique position and its ability to form four covalent bonds. Understanding the atomic structure of carbon provides valuable insights into its significance in the world of chemistry and biology.

Credit: www.britannica.com
Electrons: The Carriers Of Chemical Identity
Understanding the role of electrons is crucial in comprehending the fundamental building blocks of matter. In this context, carbon, a fundamental element in organic chemistry, serves as an excellent case study. Let’s delve into the intriguing world of electrons and their pivotal role in the chemical identity of carbon.
Electron Basics
Electrons, the smallest of the three primary particles, exist in a cloud surrounding the nucleus. They carry a negative charge and contribute significantly to the properties of an element.
Role In Chemical Bonding
The chemical bonding behavior of carbon is inherently tied to its electron configuration. With four electrons in its outer shell, carbon can form stable covalent bonds with other atoms, allowing for the vast array of organic compounds found in nature.
Decoding The Electron Count Of Carbon
Understanding the electron count of carbon is essential in unraveling the mysteries of this versatile element. Carbon, with its atomic number 6, is renowned for its ability to form a vast number of compounds, making it the backbone of organic chemistry. To comprehend how carbon achieves its remarkable properties, we need to explore its valence shell configuration and total electron tally.
Valence Shell Configuration
The valence shell configuration of carbon reveals the arrangement of electrons in its outermost shell. Carbon has four valence electrons, occupying the 2s and 2p orbitals. This configuration gives carbon the capacity to form covalent bonds with other elements, sharing electrons to achieve stability. The valence shell configuration of carbon, specifically the presence of four electrons, is what enables its ability to form a diverse array of compounds.
Total Electron Tally
When we tally up the total number of electrons in a carbon atom, we find that it possesses six electrons in total. This count includes the four valence electrons, as well as the two electrons in the inner shells. Understanding this electron count is crucial in predicting carbon’s reactivity and its ability to bond with other elements. By maintaining a balance of six electrons, carbon can form stable bonds and participate in various chemical reactions.
Overall, decoding the electron count of carbon is a fundamental step in comprehending its unique properties and its role in the world of chemistry. By understanding the valence shell configuration and total electron tally, we gain insight into the remarkable versatility of carbon and its ability to form the building blocks of life.
Credit: www.quora.com
Carbon’s Electrons And Chemical Reactivity
Carbon’s electrons play a crucial role in its chemical reactivity. Understanding the electron configuration of carbon sheds light on its ability to form covalent bonds and its impact on organic chemistry.
Formation Of Covalent Bonds
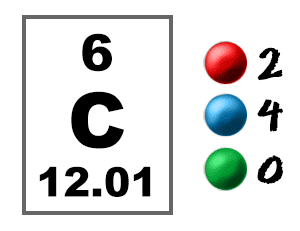
Carbon has 6 electrons, with 2 in the first shell and 4 in the second shell. This configuration allows carbon to readily form 4 covalent bonds with other atoms, leading to the formation of diverse organic compounds. These covalent bonds involve the sharing of electrons, contributing to the stability and versatility of carbon-based molecules.
Impact On Organic Chemistry
The unique electron configuration of carbon gives rise to the vast array of organic compounds found in nature and synthesized in laboratories. Carbon’s ability to form strong covalent bonds enables the creation of complex structures, such as proteins, carbohydrates, and lipids, which are essential to life. This fundamental role in organic chemistry underpins the richness and diversity of carbon-based compounds that exist in the natural world.
Comparative Analysis: Carbon Vs. Other Elements
In the realm of chemistry, carbon stands as a fundamental element, characterized by its unique electron configuration and reactivity. Let’s delve into a comparative analysis of carbon versus other elements to comprehend the distinct features.
Electron Configuration Variance
Carbon, with 6 electrons, exhibits a balanced electron configuration, allowing it to form stable covalent bonds. In contrast, elements like oxygen and nitrogen possess 8 and 7 electrons respectively, leading to contrasting reactivity patterns.
Reactivity Differences
Carbon’s reactivity is characterized by its ability to form strong bonds with other carbon atoms, resulting in the diversity of organic compounds. On the other hand, elements such as sodium and chlorine display distinct reactivity due to their electron configuration variances.
Credit: www.chem4kids.com
Visualizing Carbon’s Electrons: Models And Diagrams
Explore the world of carbon’s electrons through engaging models and diagrams. Uncover the answer to the intriguing question: How many electrons does carbon possess? Delve into the visual representation of carbon’s electron configuration for a deeper understanding.
Bohr Model Representation
The Bohr model simplifies electron arrangement around the nucleus.
Electrons orbit nucleus in distinct energy levels or shells.
Quantum Mechanical Model
The Quantum Mechanical model describes electrons as clouds around the nucleus.
Electrons have dual wave-particle nature in this model.
Applications Influenced By Carbon’s Electron Configuration
Carbon has 6 electrons in its outer shell, influencing applications in various fields. These include organic chemistry, where carbon’s electron configuration determines its bonding capabilities and the formation of diverse compounds. In materials science, carbon’s electron arrangement contributes to its unique properties, impacting its use in manufacturing and technology.
Innovations In Material Science
Carbon’s electron configuration influences material properties like strength and conductivity.Breakthroughs In Organic Synthesis
Organic synthesis advancements leverage carbon’s electron configuration for new molecule creation.Frequently Asked Questions
How Many Electrons Does A Carbon Atom Have?
A carbon atom has 6 electrons, arranged in two energy levels. The first energy level has two electrons and the second energy level has four electrons.
Why Is Carbon Important In Organic Chemistry?
Carbon is important in organic chemistry because it can form covalent bonds with other carbon atoms and with other elements like hydrogen, oxygen, and nitrogen. This ability to form a variety of stable bonds allows carbon to form the complex molecules that are essential for life.
How Is Carbon Used In Industry?
Carbon is used in industry for a variety of purposes. It is used to make steel, as a fuel in the form of coal, oil, and natural gas, and to create a variety of chemicals like plastics, fertilizers, and medicines.
How Does Carbon Affect Climate Change?
Carbon contributes to climate change when it is released into the atmosphere in the form of carbon dioxide. This happens when we burn fossil fuels like coal, oil, and gas. Carbon dioxide is a greenhouse gas that traps heat in the atmosphere and contributes to global warming.
Conclusion
Carbon is an element that has six electrons. This blog post has discussed the importance of understanding the electron structure of carbon atoms. By knowing the number of electrons in carbon, we can better understand its behavior in chemical reactions and its role in the formation of organic molecules.
Having a basic knowledge of electron structure is crucial for anyone interested in chemistry and related fields.






